close
Choose Your Site
Global
Social Media



Views: 0 Author: Site Editor Publish Time: 2025-07-02 Origin: Site








The chemical reaction from calcium to calcium oxide is simple. Calcium metal reacts with oxygen gas. The balanced equation is:
2Ca + O₂ → 2CaO
This reaction makes calcium oxide. Calcium oxide is important for many industries. There are two main ways to make calcium oxide from calcium. One way is reacting calcium with oxygen. The other way is heating calcium carbonate. The construction and steel industries use a lot of calcium oxide. It is also used to help the environment. Companies like Changshu Hongyu make products like calcium hydroxide and calcium carbonate. These products are needed more around the world.
The process of producing calcium oxide is simple and primarily involves the heating of calcium carbonate (limestone) to form calcium oxide (quicklime). This reaction is known as calcination. Although calcium metal can also react with oxygen to form calcium oxide, this is not the common industrial method. Instead, the main approach for producing calcium oxide on a large scale is by heating calcium carbonate. This method is crucial for many industries, such as construction, steel, and environmental protection.
Calcination Process and Chemical Equation
The primary chemical reaction for producing calcium oxide is as follows:
CaCO3(s)→CaO(s)+CO2(g)
In this process, calcium carbonate (CaCO₃) is heated in a kiln or furnace, where it decomposes into calcium oxide (CaO) and carbon dioxide (CO₂) gas. The high heat provided during calcination breaks down calcium carbonate into these two products.
Calcium reacts fast with oxygen and makes calcium oxide. Calcium oxide is a white powder. Many industries use this powder. The main way to get calcium oxide is by heating calcium carbonate. This process is called calcination. Calcium oxide is very important for making cement. It helps clean gases and treat water. It also helps make soil better. You need to wear safety gear when handling calcium oxide. Gloves and goggles protect your skin, eyes, and lungs. Companies like Changshu Hongyu make good calcium oxide. They use strict safety and environmental rules.

Changing calcium to calcium oxide is a simple process. This happens when calcium metal touches oxygen in the air. The equation must be balanced to show the right number of atoms. Here is how you balance the equation:
Start with the unbalanced equation: Ca + O₂ → CaO.
Count the atoms on each side. The left side has 1 calcium and 2 oxygen atoms. The right side has 1 calcium and 1 oxygen atom.
To fix the oxygen, put a 2 in front of CaO: Ca + O₂ → 2CaO.
Next, add a 2 in front of Ca: 2Ca + O₂ → 2CaO.
The final balanced equation is:
2Ca + O₂ → 2CaO
This means two calcium atoms react with one oxygen molecule to make two calcium oxide units. This is a synthesis reaction. Two elements join to make a new compound.
Calcium reacts with oxygen when it burns in air. This makes calcium oxide, which is a white powder. Many industries use this powder. The reaction gives off lots of heat and light. It is called exothermic because it releases energy. Scientists have studied how much energy this reaction gives off. The standard enthalpy of formation for calcium oxide is about -624.5 kJ/mol. This means the reaction releases a lot of energy. That is why the reaction is fast and complete.
Note: Only professionals should do this reaction. It can be dangerous because it makes a lot of heat and bright light.
The chemical reaction from calcium to calcium oxide is important for science and industry. Companies like Changshu Hongyu use this reaction to make high-purity calcium oxide. Their products, like calcium hydroxide and calcium carbonate, help many industries. These include construction, environmental protection, and medicine.
To see how this reaction compares to other ways, look at this table:
Aspect | Direct Reaction (Calcium + Oxygen) | Calcination of Calcium Carbonate |
|---|---|---|
Reaction Mechanism | Calcium reacts directly with oxygen to form CaO | Calcium carbonate decomposes to CaO and CO₂ |
Temperature Range | Occurs at high temperatures | Requires 850–1000 °C |
Efficiency | Highly efficient, fast reaction | Efficient, but depends on CO₂ pressure |
Product Reactivity | Forms calcium oxide with high purity | Produces porous CaO, enhancing reactivity |
Industrial Use | Less common for large-scale production | Main method for industrial calcium oxide |
The reaction from calcium to calcium oxide is fast and gives off a lot of energy. But most industries use the calcination of calcium carbonate to make calcium oxide in large amounts. Both ways make calcium oxide. The choice depends on the product quality and how efficient the process is.
Calcium oxide is important for making building materials and treating water. It also helps in chemical manufacturing. The reaction that makes calcium oxide is used in many other processes. These include making calcium hydroxide and light calcium carbonate. Changshu Hongyu checks every batch of calcium oxide for quality. This makes their products trusted by customers worldwide.

Calcination is an important process in factories. It changes calcium carbonate into calcium oxide. Workers heat limestone or other materials with calcium carbonate in a kiln. The heat starts a chemical reaction. In this reaction, calcium carbonate breaks down into calcium oxide and carbon dioxide gas. The reaction looks like this:
CaCO₃(s) → CaO(s) + CO₂(g)
This reaction makes quicklime, which is another name for calcium oxide. The material does not melt during this process. High heat breaks it apart. The carbon dioxide gas leaves the kiln. The solid calcium oxide stays behind. If workers do not handle the calcium oxide quickly, it can react with carbon dioxide in the air. Then it turns back into calcium carbonate. Many factories use this reaction to make a lot of calcium oxide. They use it for things like cement and steel.
Note: The calcination process is the main way to make calcium oxide in large amounts. It works better than the direct reaction of calcium with oxygen.
The calcination process needs careful control of heat and air. The reaction starts at about 640°C. It finishes near 825°C when done in air. Most factories use rotary kilns or calciners to keep the heat steady. These machines heat the material to about 850°C. This makes sure the reaction is complete.
The process works best with lots of oxygen or in a controlled space.
The kiln must keep the material from melting.
The reaction makes carbon dioxide, which must be managed to help the environment.
Modern kilns use about 3.75 gigajoules of energy for each ton of calcium oxide. They also use about 20 kilowatt-hours of electricity per ton. Companies like Changshu Hongyu use new technology to control the process. This helps make better products. They also try to lower emissions and use byproducts in smart ways. The main byproduct, carbon dioxide, is often captured or used in other jobs to protect the environment.
New ideas include using green ways to make calcium oxide nanoparticles. Automation also helps make the process better. These steps help companies follow strict environmental rules. They also make high-purity products for building, farming, and medicine. Changshu Hongyu’s skill in calcination and related products, like calcium hydroxide and calcium carbonate, helps many industries around the world.
Calcium oxide is very important in many industries. In construction, workers use it to make cement. It mixes with clay and water to make clinker. Clinker is then ground into cement powder. Builders also use it in mortar and plaster. They turn it into calcium hydroxide, which helps make strong buildings. Its high heat resistance and how it reacts with water are useful for making concrete.
It helps make mortar and concrete by reacting with water to form calcium hydroxide.
It is needed to make cement for roads, bridges, and buildings.
In the chemical industry, calcium oxide helps remove sulfur dioxide from gases. Power plants and factories use it to clean the air. It reacts with acidic gases to help meet clean air rules.
It helps control pH and neutralize bad gases.
It makes gypsum as a by-product, which is used in building.
For the environment, calcium oxide is used to treat water and soil. Lime treats animal waste, sewage, and factory sludge. It helps fix heavy metals, changes pH in wastewater, and softens water. In mining, it helps with acid mine drainage and makes soil better.
It helps clean water and fix soil, making places safer.
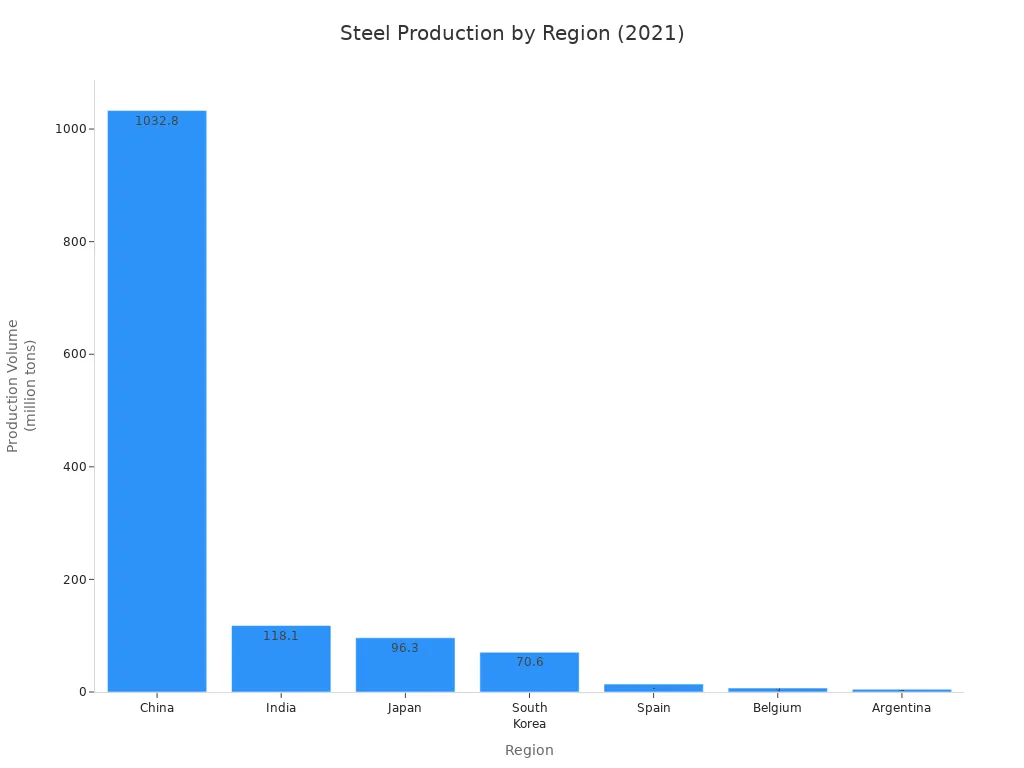
Calcium oxide is also used in making metals. It helps remove bad stuff from steel and aluminum. This makes the metal better. The chart below shows how much steel different countries make, which increases the need for calcium oxide.
People must be careful when handling calcium oxide. It can hurt your skin, eyes, and lungs. Workers should wear safety glasses to protect their eyes. They should use gloves and special clothes to keep it off their skin.
Use a respirator if there is dust or fumes.
Do not make dust and do not breathe it in.
Make sure work areas have good airflow.
Keep calcium oxide in a cool, dry place with good air. Keep containers closed tight and away from water. Wash your hands before eating or leaving work. Follow all local rules for storing and throwing it away.
Tip: Always use safety gear and follow rules when working with calcium oxide or related products like calcium hydroxide and calcium carbonate.
Changshu Hongyu makes sure their products are safe and high quality. Their calcium oxide meets rules in the U.S., Europe, and Japan. This means it can be used in food, medicine, and to help the environment.
When calcium reacts with oxygen or when calcium carbonate is heated, calcium oxide is made.
Factories use this process to make quicklime for steel, glass, and cleaning water.
Calcium oxide is also found in cement, soil fixes, and things used at home.
Companies such as Changshu Hongyu make sure their calcium oxide, calcium hydroxide, and calcium carbonate are safe and good for people everywhere.
New machines and ideas now help make these products in safer and cleaner ways, which is better for the future.
Calcium oxide is used to make cement. It also treats water and cleans gases from factories. Many steel factories use it too. Changshu Hongyu sells pure calcium oxide for these jobs.
Calcium oxide mixes with water to make calcium hydroxide. Calcium hydroxide is softer and mixes better in water. Both are important for building and helping the environment.
Calcium oxide can bother your skin, eyes, and lungs. People should wear gloves, goggles, and masks when using it. Changshu Hongyu uses strong safety rules to keep people safe and make good products.
Most factories use limestone as the main material. Limestone has calcium carbonate in it. Heating limestone makes calcium oxide and carbon dioxide. This is called calcination.
Customers can go to Changshu Hongyu’s website to buy calcium oxide and other products. The company gives good service and helps buyers all over the world.